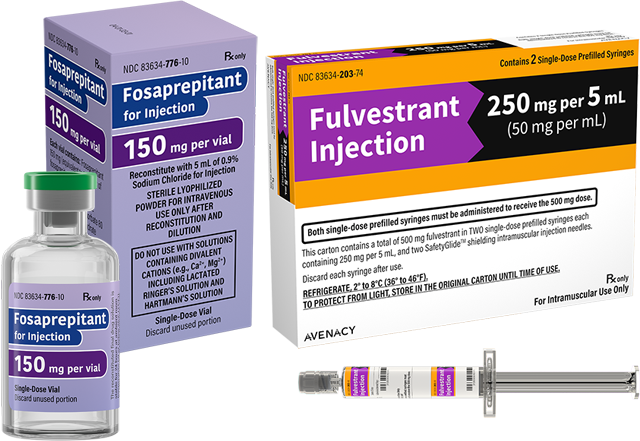
 Company adds two injectable products, Fosaprepitant for Injection and Fulvestrant Injection, to its growing portfolio for the U.S market.
Company adds two injectable products, Fosaprepitant for Injection and Fulvestrant Injection, to its growing portfolio for the U.S market.
Latest progress marks continued momentum following October 2023 launch, with four portfolio products and a growing pipeline.
Management to showcase the Company’s portfolio of critical injectable medications and differentiated business model at DCAT Week 2024 from March 18-21 in New York.
SCHAUMBURG, Ill.–(BUSINESS WIRE)–Avenacy, a specialty pharmaceutical company focused on supplying critical injectable medications, today announced it has launched Fosaprepitant for Injection and Fulvestrant Injection in the United States.
“Avenacy has had a strong start to 2024, with two product launches already executed and two more newly announced additions to our rapidly expanding portfolio. Since the Company’s launch in October, we have invested in bringing high-demand injectable products to market and strengthening our global network of development and manufacturing partners, and we are ready to build upon this important foundation as we prepare for significant growth,” said Jeff Yordon, Co-Founder and CEO of Avenacy. “We are looking forward to participating in DCAT 2024, where we will be discussing how Avenacy is well-positioned to continue addressing key gaps in the U.S. drug supply landscape through its differentiated portfolio, robust pipeline, and unwavering commitment to quality, safety, and patient care.”
Fosaprepitant for Injection is a therapeutic equivalent generic for Emend® (fosaprepitant) approved by the U.S. Food and Drug Administration. Fosaprepitant for Injection, in combination with other antiemetic agents, is indicated in adults for the prevention of:
- Acute and delayed nausea and vomiting associated with initial and repeat courses of highly emetogenic cancer chemotherapy (HEC) including high-dose cisplatin
- Delayed nausea and vomiting associated with initial and repeat courses of moderately emetogenic cancer chemotherapy (MEC).
Fosaprepitant for Injection had U.S. sales of approximately $35 million for the twelve months ending in June 2023.1 Avenacy’s product contains 150 mg of Fosaprepitant as a lyophilized powder in a single-dose vial for reconstitution.
Fulvestrant Injection is a therapeutic equivalent generic for Faslodex® (fulvestrant) approved by the U.S. Food and Drug Administration. Fulvestrant Injection is indicated for:
- Monotherapy use for the treatment of hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative advanced breast cancer in postmenopausal women not previously treated with endocrine therapy; or HR-positive advanced breast cancer in postmenopausal women with disease progression following endocrine therapy.
- Combination therapy use for the treatment of HR-positive, HER2-negative advanced or metastatic breast cancer in postmenopausal women in combination with ribociclib as initial endocrine based therapy or following disease progression on endocrine therapy; or HR-positive, HER2-negative advanced or metastatic breast cancer in combination with palbociclib or abemaciclib in women with disease progression after endocrine therapy.
Fulvestrant Injection had U.S. sales of approximately $71 million for the twelve months ending in June 2023.1 Avenacy’s product is supplied as 5 mL pre-filled syringes for injection containing 250 mg per 5 mL Fulvestrant.
Please see links for Full Prescribing Information of Fosaprepitant for Injection and Fulvestrant Injection.
In line with Avenacy’s mission to champion patient safety and streamline patient care, Fosaprepitant for Injection and Fulvestrant Injection will feature the Company’s highly differentiated packaging and labeling to support accurate medication selection. Avenacy will begin shipping Fosaprepitant for Injection and Fulvestrant Injection to wholesale partners this week.
Since launching in October of last year, Avenacy, backed by a robust global network of development and FDA-approved cGMP-certified contract manufacturing partners, has already brought four injectable products to the U.S. market. The company is poised to continue this trajectory with plans to pursue 20+ additional products for launch this year.
To learn more about Avenacy’s products or the Company’s exciting growth plans for 2024, please see the Avenacy management team at DCAT Week 2024 taking place in New York City from March 18-21st. To request a meeting with a member of the Avenacy team, please reach out to Avenacy@fticonsulting.com.
Emend® is a registered trademark of Merck & Co.
Faslodex® is a registered trademark of AstraZeneca.
1Source: IQVIA
About Avenacy
Avenacy is a U.S.-based specialty pharmaceutical company focused on supplying critical injectable medications used to treat patients in various medically supervised settings, from acute care hospitals to outpatient clinics and physician offices. Through a rigorous and optimized selection process, the Company is building out a pipeline of high-quality FDA approved injectable products in order to ensure a resilient portfolio that can meet the needs of today’s dynamic drug supply chain. With an experienced team, commitment to quality and reliability, and product offerings intended to facilitate safe and efficient patient care, Avenacy strives to be a trusted partner for essential medications.
Avenacy was launched in 2023 and is headquartered in Schaumburg, IL. For more information, please visit https://www.avenacy.com.
Media Contact
FTI Consulting Avenacy@fticonsulting.com