Furosemide Injection, USP
PLEASE SEE FULL PRESCRIBING INFORMATION, INCLUDING BOXED WARNING.
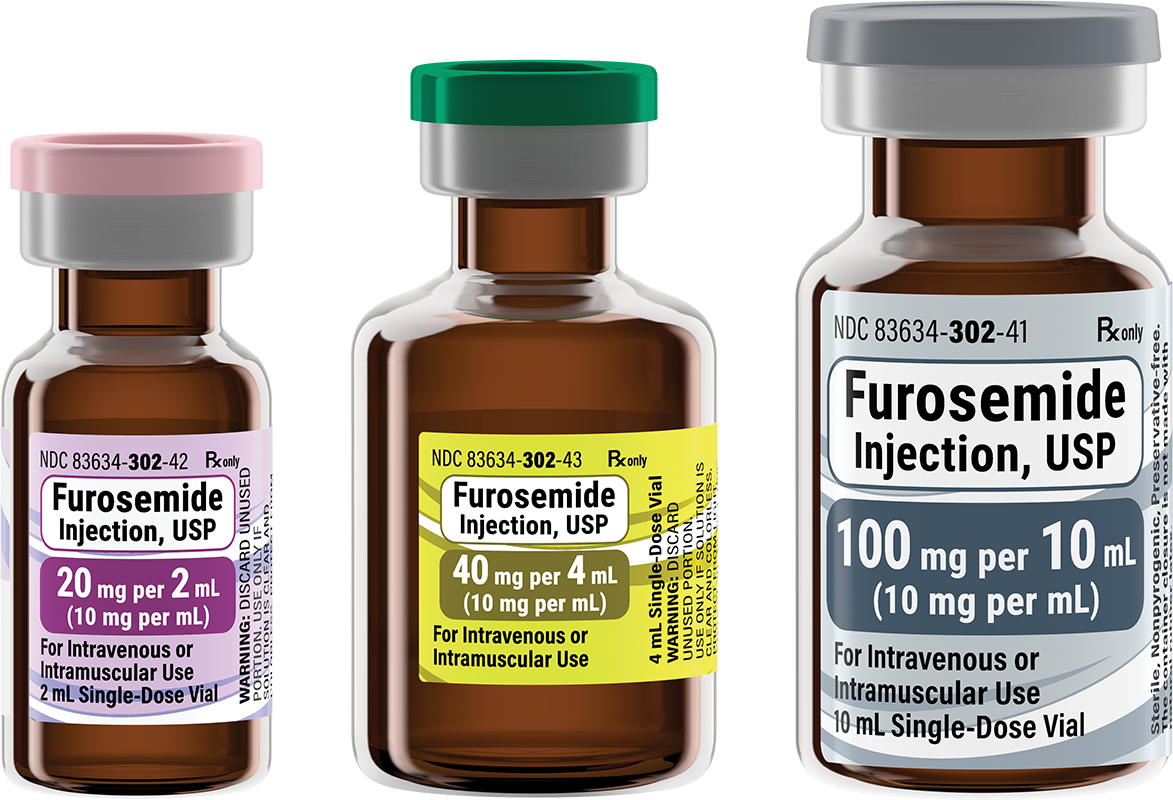
NDC Number
(Unit of Sale)
(Unit of Sale)
Strength
20 mg per 2 mL
40 mg per 4 mL
100 mg per 10 mL
Concentration
10 mg per mL
10 mg per mL
10 mg per mL
Description
- Single-Dose Vial
- Single-Dose Vial
- Single-Dose Vial
Closure
13 mm
13 mm
20 mm
Barcoded
Unit of Sale
25 Vials
25 Vials
25 VIals
Storage
Room Temperature
Room Temperature
Room Temperature
Benefits
- AP-Rated
- Preservative-Free
- Not Made With Natural Rubber Latex
- AP Rated
- Preservative-Free
- Not Made With Natural Rubber Latex
- AP Rated
- Preservative-Free
- Not Made With Natural Rubber Latex
Prescribing and Safety Information
NDC Number
(Unit of Sale)
(Unit of Sale)
Strength
20 mg per 2 mL
Concentration
10 mg per mL
Description
- Single-Dose Vial
Closure
13 mm
Barcoded
Unit of Sale
25 Vials
Storage
Room Temperature
Benefits
- AP-Rated
- Preservative-Free
- Not Made With Natural Rubber Latex
Prescribing and Safety Information
NDC Number
(Unit of Sale)
(Unit of Sale)
Strength
40 mg per 4 mL
Concentration
10 mg per mL
Description
- Single-Dose Vial
Closure
13 mm
Barcoded
Unit of Sale
25 Vials
Storage
Room Temperature
Benefits
- AP Rated
- Preservative-Free
- Not Made With Natural Rubber Latex
Prescribing and Safety Information
NDC Number
(Unit of Sale)
(Unit of Sale)
Strength
100 mg per 10 mL
Concentration
10 mg per mL
Description
- Single-Dose Vial
Closure
20 mm
Barcoded
Unit of Sale
25 VIals
Storage
Room Temperature
Benefits
- AP Rated
- Preservative-Free
- Not Made With Natural Rubber Latex
Prescribing and Safety Information


